1. Medical Device Support Programs and Agencies
1) Medical Device Industry Vision 2024 (Ministry of Economy, Trade and Industry)
The Ministry of Economy, Trade and Industry (METI) inaugurated the Medical Device Industry Vision Study Group, which brings together representatives from industry, academia and government, aiming to hold discussions on the future direction that Japanese companies should aim for amid the dramatic changes in the medical device industry and the kind of support METI is expected to provide to the industry. The study group held repeated discussions on the future direction that the medical device industry should aim for, necessary support measures, and strategic efforts toward implementing such measures, and compiled the results of the discussions into the Medical Device Industry Vision 2024.
○ Direction to aim for and necessary support measures
The study group has set out the direction for the medical device industry to grow into a high-value-added industry as industrial growth through a cycle of R&D investment to create innovation and investment recovery through global expansion, and has outlined the following four initiatives as strategic efforts to achieve this.
[Creating companies that will take steps to expand globally, including in the United States]
1) Support for strengthening competitiveness and building networks by building evidence for expansion into the US
2) Support for creating an environment and building a network that facilitates global expansion
[Creating a research and development environment that fosters innovation]
3) Promoting the development of medical devices using digital technologies such as AI
4) Strengthening collaboration and acceleration between major companies and startups
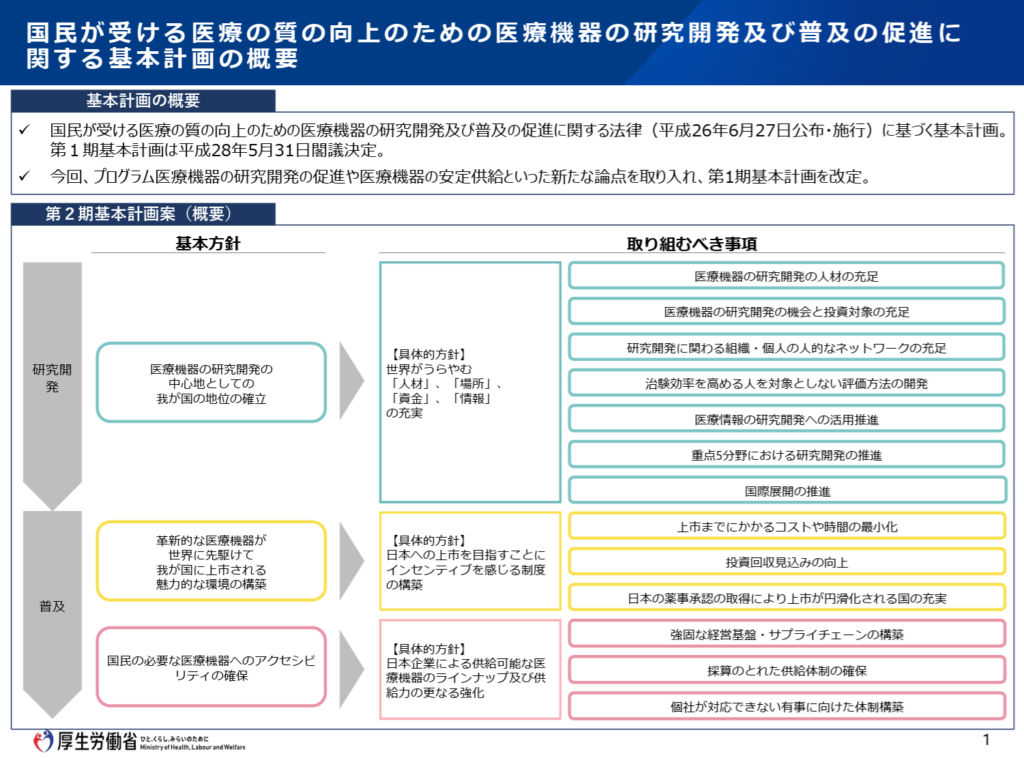
2) Second Phase of the Basic Plan for Medical Devices (Ministry of Health, Labour and Welfare)

3) Next-generation Health Tech Startup Development Support Project (AMED)
We aim to create startup companies that develop innovative health tech by providing support for research and development funds to researchers and researchers from academia and private companies who are aiming to start businesses in the health tech field, as well as providing support for individual research and development issues through an accompanying support consortium.
4) Medical and Engineering Collaboration Innovation Promotion Project (AMED)
◆Development and commercialization projects (subsidized projects)
The consortium will solicit proposals for commercializing medical devices, etc., and then decide on the projects to be selected. The selected consortium will then proceed with the development of the equipment, including prototype development, mass production trials, non-clinical trials, and clinical evaluations, while also making preparations for commercialization, such as obtaining licenses and permits, intellectual property, sales and logistics strategies, and establishing systems.
In this process, we provide support, primarily consulting on permits, commercialization, intellectual property, and technical aspects, and provide support for commercialization.
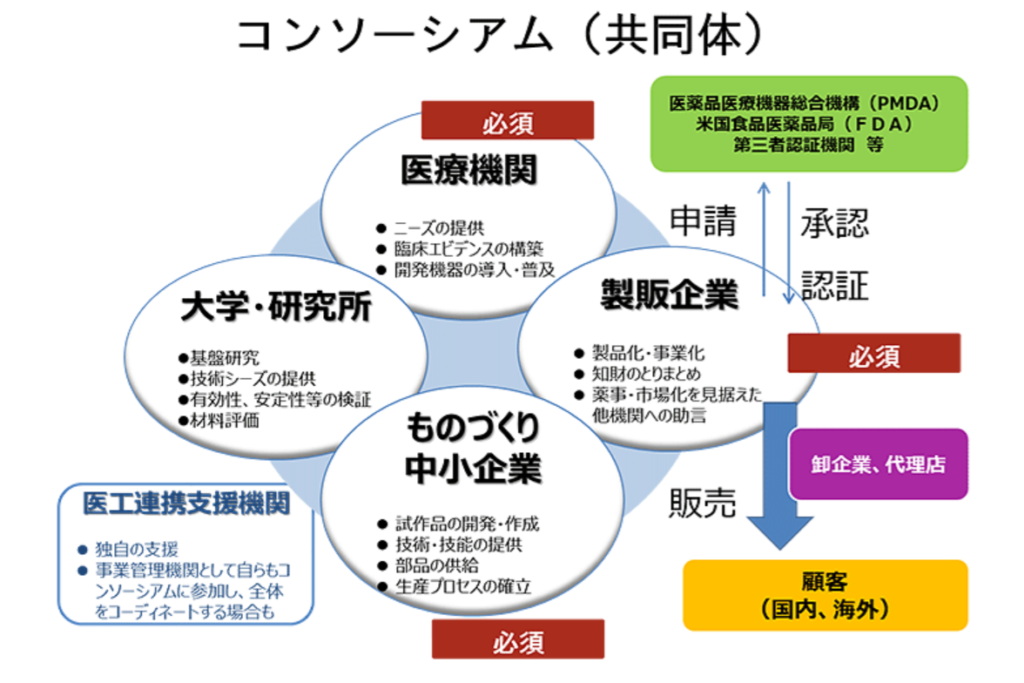
◆Development and commercialization business (venture development) (commissioned business)
In order to encourage venture companies to enter the market, we will support early-stage initiatives (proof of concept, etc.) that are difficult for venture capitalists to handle.
◆Medical Device Development Support Network Project (Commissioned Project)
With a view to contributing to future medical device policies, we will promote a national and regional collaborative network that will enable medical device developers, including new entrants, to receive comprehensive support in a wide range of areas, including licensing, intellectual property, technical aspects, and marketing, so that the development and commercialization of medical devices through medical-engineering collaboration can progress autonomously.

◆Regional Collaboration Center Independence Promotion Project (Commissioned Project)
We will promote the formation of a medical device development ecosystem in the region by deploying commercialization personnel with specialized knowledge in medical device development, promoting matching of seeds with needs in the region, and facilitating commercialization.
5) Medical Device Development Promotion Research Project (AMED)
This research project aims to provide safer medical technologies to the public as soon as possible, and to link the results of basic research produced in Japan to the approval of medical devices under the Act on Ensuring Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, etc. (hereinafter referred to as the Pharmaceutical and Medical Device Act). In order to do so, the project will promote high-quality clinical research and investigator-initiated clinical trials that have a high probability of practical application and can fully guarantee scientific and ethical standards.
We support clinical research and investigator-initiated clinical trials aimed at creating innovative medical devices on the following themes.
- Clinical research and investigator-initiated clinical trials for medical devices that contribute to reducing the burden of medical care
- Non-clinical research (appropriate confirmatory testing to replace clinical trials) aimed at commercializing innovative medical devices, clinical research, and investigator-initiated clinical trials
- Clinical research and investigator-initiated clinical trials aimed at commercializing pediatric medical devices
- Clinical research and investigator-initiated clinical trials aimed at commercializing medical devices for the elderly
- Development of medical devices using disease registration systems (registries)
6) Medical Engineering Global Collaboration Project (Global Expansion Base Project) (AMED)
The purpose of this initiative is to realize the basic principles of the “Health and Medical Strategy” (Cabinet decision on March 27, 2020, partially revised on April 9, 2021), which are to “contribute to the provision of medical care using the world’s most advanced technology” and “contribute to economic growth.”
We aim to support the development of innovative medical devices by small and medium-sized enterprises with advanced manufacturing technology and startups with cutting-edge ideas, with the aim of developing medical devices that will be used in medical settings not only in Japan but around the world. Furthermore, we aim to not only provide support in providing knowledge of pharmaceutical regulations, etc., necessary for medical device development, but also to revitalize the domestic medical device industry by providing the support necessary for international expansion.
This project will provide development support to small and medium-sized enterprises and startups by placing commercialization personnel with specialized knowledge in medical device development at regional collaboration hubs, which will serve as the core of the medical device development ecosystem, and by promoting the matching of seeds and needs scattered throughout the region and the commercialization of medical device development with an eye toward utilization in global standard treatments.












